Magnesium Nitride Charge On Ion . metals form positive ions (cations). to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. to get a +2 charge, the ion had to lose two electrons. the symbol for the ion is mg 2+, and it is called a magnesium ion. A magnesium atom must lose two electrons to have the same number electrons as an. 3 mg + n2 → mg3n2. Or ammonia at 700 °c: (b) since n 3− is an anion, its. By passing dry nitrogen over heated magnesium at 800 °c: A magnesium ion therefore has 10 electrons.
from www.chegg.com
(b) since n 3− is an anion, its. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. A magnesium ion therefore has 10 electrons. Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. to get a +2 charge, the ion had to lose two electrons. 3 mg + n2 → mg3n2. By passing dry nitrogen over heated magnesium at 800 °c: metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. Or ammonia at 700 °c:
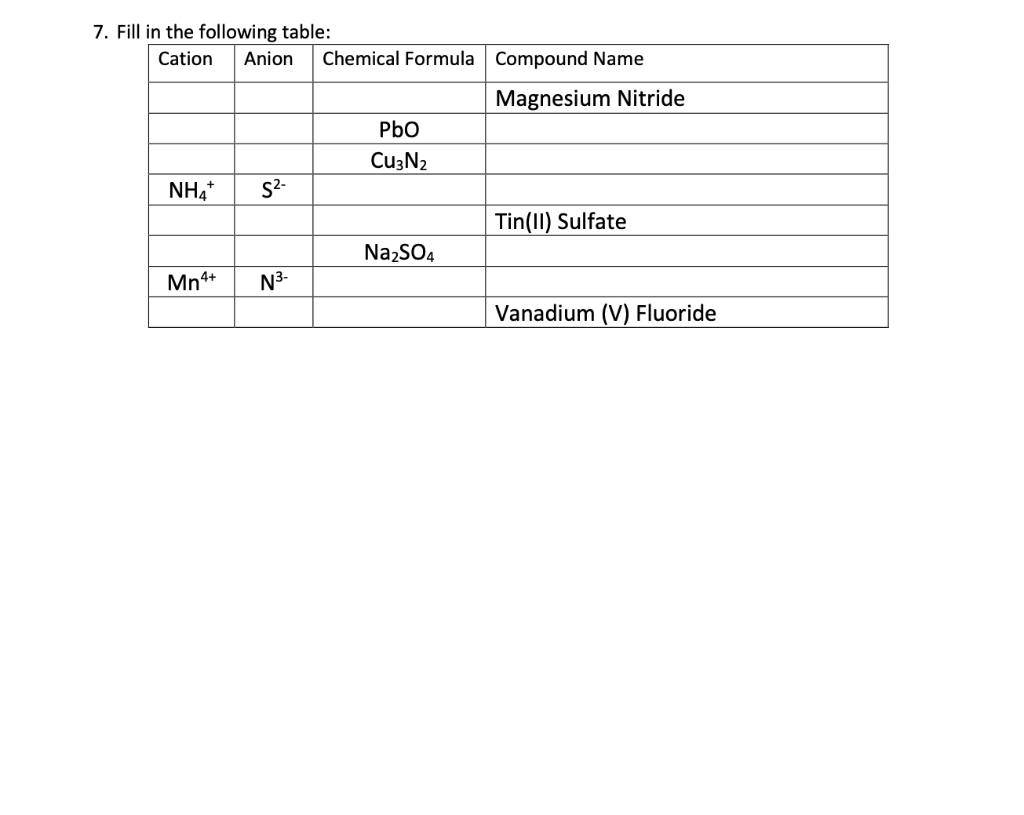
Solved 7. Fill in the following table Cation Anion Chemical
Magnesium Nitride Charge On Ion Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. Or ammonia at 700 °c: A magnesium atom must lose two electrons to have the same number electrons as an. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. By passing dry nitrogen over heated magnesium at 800 °c: the symbol for the ion is mg 2+, and it is called a magnesium ion. to get a +2 charge, the ion had to lose two electrons. (b) since n 3− is an anion, its. A magnesium ion therefore has 10 electrons. 3 mg + n2 → mg3n2. metals form positive ions (cations).
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation, free download ID Magnesium Nitride Charge On Ion to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. By passing dry nitrogen over heated magnesium at 800 °c: Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. 3 mg + n2 → mg3n2. the symbol for. Magnesium Nitride Charge On Ion.
From www.youtube.com
Nitride, Nitrite, and Nitrate Ions (Difference and Formulas) YouTube Magnesium Nitride Charge On Ion metals form positive ions (cations). Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. (b) since n 3− is an anion, its. By passing dry nitrogen over heated. Magnesium Nitride Charge On Ion.
From www.elementschina.com
Magnesium Nitride Mg3N2 CAS No.12057715 Elements China Magnesium Nitride Charge On Ion to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. A magnesium ion therefore has 10 electrons. A magnesium atom must lose two electrons to have the same number electrons as an. By passing dry nitrogen over heated magnesium at 800 °c: Nitrogen’s position in the. Magnesium Nitride Charge On Ion.
From www.scienceforums.net
How to count formal charge in Nitrite Ion? Chemistry Magnesium Nitride Charge On Ion A magnesium ion therefore has 10 electrons. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. 3 mg + n2 → mg3n2. Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. (b) since n 3− is an anion,. Magnesium Nitride Charge On Ion.
From www.youtube.com
Equation for Mg(NO3)2 + H2O (Magnesium nitrate + Water) YouTube Magnesium Nitride Charge On Ion metals form positive ions (cations). Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. (b) since n 3− is an anion, its. A magnesium atom must lose two electrons to have the same number electrons as an. 3 mg + n2 → mg3n2. to balance the charges with the lowest number. Magnesium Nitride Charge On Ion.
From www.numerade.com
SOLVED Write the balanced equation for Magnesium nitride + water Magnesium Nitride Charge On Ion the symbol for the ion is mg 2+, and it is called a magnesium ion. 3 mg + n2 → mg3n2. Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. metals form positive ions (cations). to get a +2 charge, the ion had to lose two electrons. A magnesium ion. Magnesium Nitride Charge On Ion.
From www.oceanproperty.co.th
N3 Lewis Structure How To Draw The Lewis Structure For, 41 OFF Magnesium Nitride Charge On Ion to get a +2 charge, the ion had to lose two electrons. the symbol for the ion is mg 2+, and it is called a magnesium ion. Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. to balance the charges with the lowest number of ions possible, we need to. Magnesium Nitride Charge On Ion.
From www.numerade.com
SOLVED Magnesium and nitrogen react in a combination reaction to Magnesium Nitride Charge On Ion the symbol for the ion is mg 2+, and it is called a magnesium ion. Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. A magnesium ion therefore has 10 electrons. (b) since n 3− is an anion, its. to get a +2 charge, the ion had to lose two electrons.. Magnesium Nitride Charge On Ion.
From www.numerade.com
SOLVED Determine the charge on each ion in the following compounds Magnesium Nitride Charge On Ion to get a +2 charge, the ion had to lose two electrons. A magnesium ion therefore has 10 electrons. metals form positive ions (cations). Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. By passing dry nitrogen over heated magnesium at 800 °c: 3 mg + n2 → mg3n2. A magnesium. Magnesium Nitride Charge On Ion.
From www.researchgate.net
Magnesium (Mg) in gallium nitride (GaN) exhibits behavior... Download Magnesium Nitride Charge On Ion to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. (b) since n 3− is an anion, its. Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. By passing dry nitrogen over heated magnesium at 800 °c: the. Magnesium Nitride Charge On Ion.
From h-o-m-e.org
What Is The Magnesium Nitride Formula? Magnesium Nitride Charge On Ion By passing dry nitrogen over heated magnesium at 800 °c: (b) since n 3− is an anion, its. Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. A magnesium. Magnesium Nitride Charge On Ion.
From www.youtube.com
Nitride ion electron configuration YouTube Magnesium Nitride Charge On Ion Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. (b) since n 3− is an anion, its. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. metals form positive ions (cations). A magnesium atom must lose two. Magnesium Nitride Charge On Ion.
From slideplayer.com
Ionic Compounds Naming and Writing Formulas ppt download Magnesium Nitride Charge On Ion metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. By passing dry nitrogen over heated magnesium at 800 °c: Or ammonia at 700 °c: 3 mg + n2 → mg3n2. (b) since. Magnesium Nitride Charge On Ion.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Magnesium Nitride Charge On Ion (b) since n 3− is an anion, its. 3 mg + n2 → mg3n2. A magnesium atom must lose two electrons to have the same number electrons as an. Or ammonia at 700 °c: to get a +2 charge, the ion had to lose two electrons. By passing dry nitrogen over heated magnesium at 800 °c: A magnesium ion. Magnesium Nitride Charge On Ion.
From www.youtube.com
Lewis Structure of AlN, Aluminum Nitride YouTube Magnesium Nitride Charge On Ion metals form positive ions (cations). to get a +2 charge, the ion had to lose two electrons. Nitrogen’s position in the periodic table ( group 15 ) reveals that it is a nonmetal. the symbol for the ion is mg 2+, and it is called a magnesium ion. By passing dry nitrogen over heated magnesium at 800. Magnesium Nitride Charge On Ion.
From itlessoneducation.com
Na3N ionic compound name All you need to know about it It Lesson Magnesium Nitride Charge On Ion By passing dry nitrogen over heated magnesium at 800 °c: the symbol for the ion is mg 2+, and it is called a magnesium ion. to get a +2 charge, the ion had to lose two electrons. A magnesium atom must lose two electrons to have the same number electrons as an. A magnesium ion therefore has 10. Magnesium Nitride Charge On Ion.
From liondigitalartillustrations.blogspot.com
draw the lewis dot structure for mg2+ liondigitalartillustrations Magnesium Nitride Charge On Ion Or ammonia at 700 °c: metals form positive ions (cations). A magnesium ion therefore has 10 electrons. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. to get a +2 charge, the ion had to lose two electrons. (b) since n 3− is. Magnesium Nitride Charge On Ion.
From www.showme.com
Magnesium nitride empirical formula Science ShowMe Magnesium Nitride Charge On Ion A magnesium atom must lose two electrons to have the same number electrons as an. 3 mg + n2 → mg3n2. to get a +2 charge, the ion had to lose two electrons. Or ammonia at 700 °c: metals form positive ions (cations). By passing dry nitrogen over heated magnesium at 800 °c: the symbol for the. Magnesium Nitride Charge On Ion.